|
|
NOx Sensitised Oxidation of
Methane
Experiments and Modelling
|
|
||
|
|
Anders Broe Bendtsena, Peter Glarborga, Kim
Dam-Johansena, Per Gravers Kristensenb & Bent Karllb |
|
||
|
Poster presented at 27th
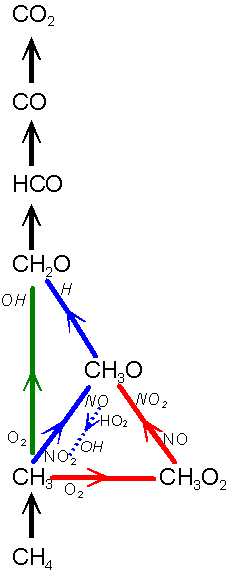
International Symposium on Combustion, Boulder, CO, USA, Aug. 3-7th 1998 Background As the initiation
processes of methane oxidation take place significantly faster we have made
experiments with shorter residence times. Furthermore we have investigated
the effect of respectively NO and NO2. Based on these experiments,
we have modified an existing mechanism to cover the experimental range.
|
|
|
||
Experimental
results
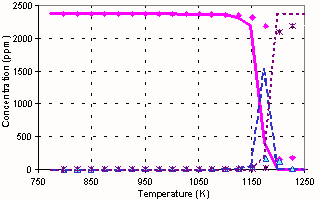
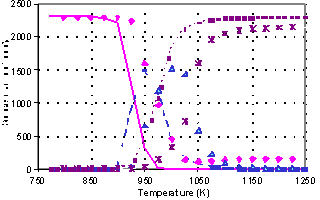
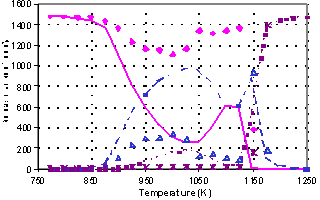
We have made several experiments in the form of a temperature scan of various
chemical compositions of the inlet to the plug flow reactor used in several
studies from our laboratory 2. Three characteristic observations
were made:
1) NO and NO2
reduces the ignition temperature as shown by Bromly et al 1;
2) At short residence times
(<150 ms) NO alone does not show this enhancing effect
3) In some experiments
partial oxidation was seen at low temperatures and full oxidation was observed
at high temperatures; but in an intermediate temperature range no oxidation was
seen.
|
|
|
|
Oxidation of methane in the
absence of NOx (200 ms) |
Oxidation of methane in the
presence of 200 ppm NO (200 ms) |
|
|
|
|
Oxidation of methane in the
presence of 200 ppm NO2 (200 ms) |
Oxidation of methane in the
presence of 200 ppm NO (140 ms) |
|
|
Experimental
conditions: NO and NO2 concentrations as specified |
|
|
Mechanistic studies References Acknowledgments |
![]() to Anders Broe Bendtsen's home page
to Anders Broe Bendtsen's home page
Last updated: February18th
2001